Introduction
Statements of conformity and decision rules are two issues that have become a topic of discussion since the revision of the ISO/IEC 17025 standard. The new requirements have become more demanding, assessors are writing deficiencies, and people have a lot of questions about how to comply.
So far, accreditation bodies are providing training on this topic and the ILAC G8 guide has been updated to help labs meet requirements. However, the problem is the training and guides that are currently available are far more advanced than what most laboratories need to meet customer and ISO/IEC 17025 requirements.
In fact, I get questions and complaints on this topic all the time.
Therefore, I created this guide to help you meet ISO/IEC 17025 requirements in the simplest way possible. You do not need advanced analyses or complex software to evaluate every measurement result. However, if you want the advanced techniques, check out my guide on taking uncertainty into account using guard banding methods.
So, before you spend time and money worrying about it, give this guide read. I am going to teach you how to meet the requirements whether you want to:
- Provide statements of conformity,
- Not provide statements of conformity,
- Take measurement uncertainty into account, or
- Not take measurement uncertainty into account.
You have options that many people will not tell you about. However, I am going to share with you what I see other labs doing that works.
If you are ready to get started, let’s dive in.
Statements of Conformity
Before you think about decision rules, you need to first decide how you are going to handle statements of conformity.
In this section, you are going to learn all about statements of conformity;
- What is a statement of conformity,
- Types of statements of conformity,
- Commonly used statements of conformity,
- How to determine conformity, and
- How to meet ISO/IEC 17025:2017 requirements
What is Conformity
Before diving into statements of conformity, let’s first define what is conformity.
According to the Oxford Lexico Dictionary, conformity is compliance with standards, rules, or laws.

Amongst all the dictionaries and definitions that I read regarding conformity, I liked this definition the best. I believed that it clearly explained how we (laboratories) should define conformity; we determine conformity based on compliance with standards, specifications, and rules.
What is a Statement of Conformity
A conformity statement or a statement of conformity is an expression that clearly describes the state of compliance or non-compliance to a specification, standard, or requirement.
I found it odd that this term is not defined in the ISO/IEC 17025:2017 or the ILAC G8:09/2019. So, I developed the definition above to give you a description of a statement of conformity.
This topic is not new, but with the revision of the ISO/IEC 17025 it has become popular due to the additional requirements to take risk into account when you provide statements in your test and calibration reports.
So, let’s learn more about statements of conformity.
Common Examples of Conformity Statements
Below is a list of conformity statements commonly used in test and calibration reports:
- Pass / Fail
- In Tolerance / Out of Tolerance
- In Spec / Out of Spec
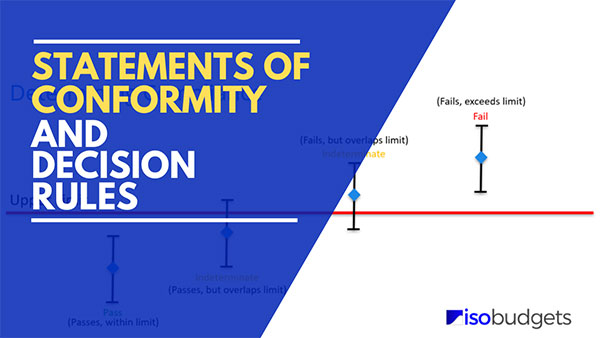
Types of Statements of Conformity
While we are on the topic of statements of conformity, it is important to review the four most common types of conformity used in accredited test and calibration reports.
The three most common types of conformity are:
- Compliance
- Non-compliance
- Conditional Compliance
- Conditional Non-compliance
Compliance
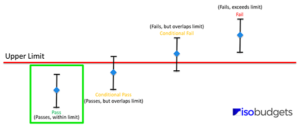
When the measurement result plus or minus the expanded uncertainty (95% C.L.) does not exceed the specification limit, then compliance with specification can be stated.
Common Examples of Compliance Statements
Below is a list of commonly used statements of compliance:
- Compliance
- Pass
- In Spec
- Within Specifications
- In Tolerance
Non-Compliance
When the measurement result plus or minus the expanded uncertainty (95% C.L.) exceeds the specification limit, then non-compliance with specification can be stated.
Common Examples of Non-Compliance Statements
Below is a list of commonly used statements of non-compliance:
- Non-Compliance
- Fail
- Out of Spec
- Outside Specifications
- Out of Tolerance
- Exceeds Limits
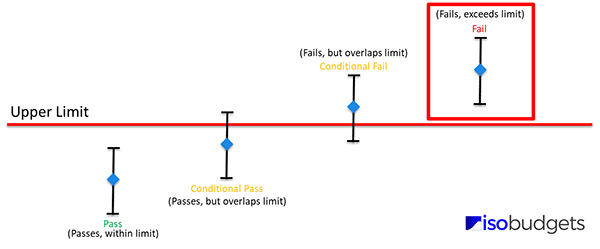
Conditional Compliance
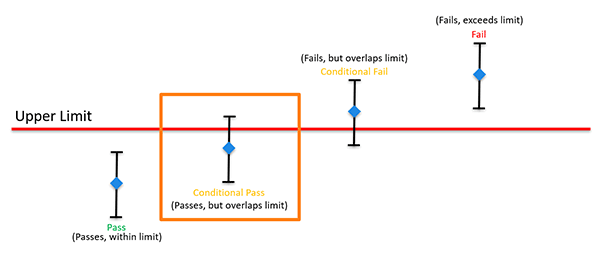
When the measurement result plus or minus the expanded uncertainty (95% C.L.) does not exceed but overlaps the specification limit, it is not possible to state compliance or non-compliance. Therefore, the result is conditional compliance.
Common Examples of Indeterminate Statements
- Conditional Pass
- Pass with overlap
Conditional Non-Compliance
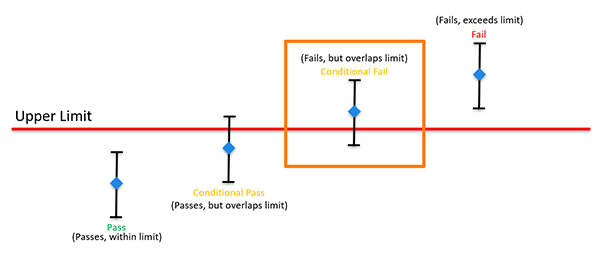
When the measurement result plus or minus the expanded uncertainty (95% C.L.) exceeds but overlaps the specification limit, it is not possible to state compliance or non-compliance. Therefore, the result is conditional non-compliance.
Common Examples of Conditional Non-compliance Statements
- Conditional Fail
- Fail with overlap
How to Determine Compliance
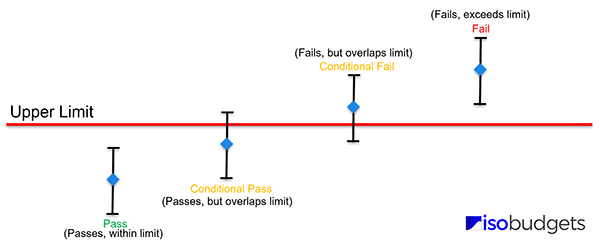
To determine compliance and provide a statement of conformance in your test or calibration reports, follow the steps listed below.
- Find your result, expanded uncertainty, and specifications;
- Add & Subtract the results and the expanded uncertainty;
- Evaluate and determine conformance:
- Pass: Result ± expanded uncertainty within limits,
- Fail: Result ± expanded uncertainty exceeds limits,
- Conditional Pass: Result ± expanded uncertainty within but overlaps limits,
- Conditional Fail: Result ± expanded uncertainty exceeds but overlaps limits.
If you notice, the key is to determine whether the result plus or minus the expanded uncertainty is within, exceeds, or overlaps the limits. By focusing on these three outcomes, you should be able to easily determine which statement of conformity is right for your results.
Meeting ISO/IEC 17025 Requirements
Statement of conformity is mentioned several times in the 2017 version of the ISO/IEC 17025 standard. Here is a list of the related requirements:
- Section 6.2.6b
- Section 7.1.3
- Section 7.8.3.1b
- Section 7.8.4.1e
- Section 7.8.6.1
- Section 6.4.5
- Section 6.4.13c
Section 6.2.6b
ISO/IEC 17025:2017 Requirement

b) analysis of results, including statements of conformity or opinions and interpretations;”
The Key Takeaway
In section 6.2.6b, personnel must be authorized to analyze results including statements of conformity.
How to Meet the Requirement
Make sure to authorize personnel to analyze results, including statements of conformity, and include a qualification in their personnel records.
Section 7.1.3
ISO/IEC 17025:2017 Requirement

The Key Takeaway
In section 7.1.3, when a customer requests statements of conformity, the following information must be clearly defined;
- The specification or standard, and
- The decision rule.
How to Meet the Requirement
If a customer requests a statements of conformity, make sure to clearly define the specification or standard and the decision rules to be used in your quotes, contracts, proposals, etc. You can easily meet this requirement by adding this information in your quotes disclaimer statement, terms and conditions, notes, comments, remarks, etc. Just make sure that it is documented in there.
Otherwise, I have observed other laboratories sending their customers an acknowledgement form that must be signed and returned.
If you are an internal test or calibration laboratory, where your company is the customer, then use a service or operating agreement to establish how the lab will use statements of conformity and decision rules.
Use the option that works best for your laboratory.
Section 7.8.3.1b
ISO/IEC 17025:2017 Requirement

b) where relevant, a statement of conformity with requirements or specifications;”
The Key Takeaway
In section 7.8.3.1, test reports must include a statement of conformity, where:
- Necessary for the interpretation of results, and
- Relevant
How to Meet the Requirement
If statements of conformity are relevant and necessary for the interpretation of results, make sure to include them in your test reports. If they are not, do not include them.
Section 7.8.4.1e
ISO/IEC 17025:2017 Requirement
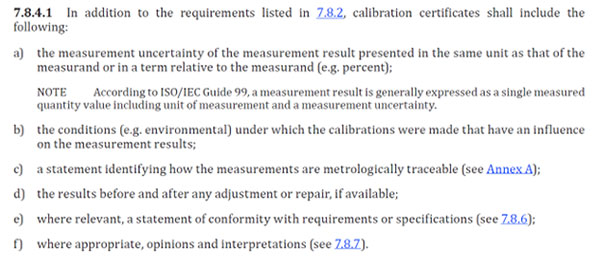
e) where relevant, a statement of conformity with requirements or specifications;”
The Key Takeaway
In section 7.8.4.1, calibration certificates must include a statement of conformity, where relevant.
How to Meet the Requirement
If statements of conformity are relevant (to your customer, laboratory, etc.), make sure to include them in your calibration certificates. If they are not, do not include them.
Section 7.8.6.1
ISO/IEC 17025:2017 Requirement

The Key Takeaway
In section 7.8.6.1, when a statement of conformity is provided, you must:
- Document the decision rule used,
- Take into account the level of risk,
- Apply the decision rule.
How to Meet the Requirement
If you provide statements of conformity in your test or calibration reports, make sure to include the required information in your certificates.
Include your decision rules in the disclaimer, notes, or comments section of your test or calibration certificate.
Take measurement uncertainty (i.e. level of risk) into account when determining conformity.
Make sure to follow the decision rules that you have documented.
Section 7.8.6.2
ISO/IEC 17025:2017 Requirement

a) to which results the statement of conformity applies;
b) which specifications, standards or parts thereof are met or not;
c) the decision rule applied.”
The Key Takeaway
In section 7.8.6.2, when a statement of conformity is provided, you must report on the statement to clearly identify;
- Which results the statement of conformity applies,
- Which specifications, standards or parts of the specification are met or not met,
- The decision rule applied.
How to Meet the Requirement
If you provide statements of conformity in your reports, make sure your conformity statements clearly show which results they apply to. Typically, this is not a problem for most labs because statements of conformity (e.g. Pass or Fail) are usually reported alongside each result.
Additionally, make sure to provide information on the specifications or standard in your certificates. Again, this is typically not a problem since most labs report specifications or limits with their measurement results.
Finally, make sure that your decision rules are provided in your test or calibration certificates. These can be included in the disclaimer, notes, or comments section of your test or calibration certificates.
Section 6.4.5
ISO/IEC 17025:2017 Requirement

The Key Takeaway
In section 6.4.5, you need to review your equipment’s calibration reports and verify it meets your measurement accuracy and(or) uncertainty requirements needed for you to perform further testing or calibration.
The problem is your calibration service provider is mostly likely using Simple Acceptance (i.e. stating Pass or Fail without taking uncertainty into account). Now, this puts the burden on you to review your calibration reports and take uncertainty into account.
If you are not verifying your equipment meets your accuracy or uncertainty requirements, then you could get a deficiency that costs you a significant amount of time to evaluate every single one of your calibration reports.
This requirement is not covered in the ILAC G8. However, many assessors are checking this and writing deficiencies for section 6.4.5. Do not fall victim to this. Make sure you are checking your equipment calibration reports.
How to Meet the Requirement
If your calibration supplier is using simple acceptance, then make sure you review and evaluate your calibration reports to confirm your equipment meets specifications when taking uncertainty into account. Furthermore, make sure to create objective evidence to prove you are performing this evaluation. You can create a form, an Excel calculator, or add a policy or procedure to perform this evaluation. You have options. Just make sure you have objective evidence to prove it.
Section 6.4.13c
ISO/IEC 17025:2017 Requirement

The Key Takeaway
Supporting section 6.4.5, section 6.4.13c requires evidence that you verified your equipment conforms to specifications. So, if you calibration service provider did not take uncertainty into account, now you have to provide evidence that you did.
How to Meet the Requirement
Review your calibration reports. If your calibration laboratory determined pass or fail taking uncertainty into account, make sure the tolerances used meet your requirements. If your calibration laboratory used simple acceptance, make sure the tolerance meet your requirements and evaluate your results taking uncertainty into account.
With regard to evidence, it is up to you. You can keep a spreadsheet of the calculations, use an evaluation form, or you could add a note to the record in your software or tracking spreadsheet. Otherwise, you could mark your calibration reports with a note, such as “Fit for use,” then date and initial it. Whichever method you decide to use, make sure keep evidence of the verification
In the next section, you are going to learn everything that you need to know about decision rules.
Decision Rules
Decision rules is the new requirement of the ISO/IEC 17025 standard that has a lot of people confused. Don’t let it intimidate you!
You simply need to describe how you decide whether a result passes or fails.
In this section, you will learn:
- What are decision rules,
- How to meet ISO/IEC 17025 requirements,
- What options you have with decision rules, and
- Examples of decision rules in use.
What are Decision Rules

According to ISO/IEC 17025:2017, section 3.7, a decision rule is a rule that describes how measurement uncertainty is accounted for when stating conformity with a specified requirement.
As you can see, the definition is very clear. What are your rules for taking measurement uncertainty into account when providing statements of conformity. How you determine whether a result passes or fails.
Meeting ISO/IEC 17025 Requirements
Section 7.8.6.1
ISO/IEC 17025:2017 Requirement

The Key Takeaway
According to section 7.8.6.1 of the ISO/IEC 17025 standard, when a statement of conformity is provided, the laboratory shall:
- Document the decision rule used,
- Take into account the level of risk (i.e. uncertainty), and
- Apply the decision rule.
How to Meet the Requirement
To meet this requirement, you need to simply document a set of rules that describes how you:
- take measurement uncertainty into account when determining conformity, and
- decide whether a result Passes or Fails
Then, apply those rules when providing statements of conformity. The key here is “when providing statements of conformity.” If you do not provide statements of conformity, then you do not need to include this information in your reports.
In the next section, you will see how other accredited laboratories are using decision rules and statements of conformity.
Decision Rule Options
When documenting, applying, and reporting decision rules, you have a few options. The three options listed below are actual real-world applications used by laboratories accredited to the latest version of the standard.
Most accredited laboratories use one of the following three options:
- Take uncertainty into account when making conformity statements,
- Do not take uncertainty into account when making conformity statements, or
- Do not make conformity statements.
Decision Rule Examples for ISO 17025
Now that you have been given options to document and apply decision rules, let’s look at some real-world examples of each option in use.
In this section, you will see three decision rule examples;
- Taking uncertainty into account,
- Not taking uncertainty into account, and
- Not providing statements of conformity.
Example 1: Taking Uncertainty into Account (ILAC G8 Decision Rule)
In this example, you will see how Keysight Technologies documents and applies decision rules and provides statements of conformity in their calibration certificates. Keysight does a great job meeting the ISO/IEC 17025:2017 requirements and implementing a process that I believe was intended by the standard.
Each Keysight calibration report provides a section that gives a list of the statements of conformity used in their reports and describes their decision rules when making these statements.
If you are looking to meet requirements and implement a similar process, this is an example that you will want to see.
Look at the exert from a Keysight calibration report to see how to they document and apply decision rules and statements of conformity.
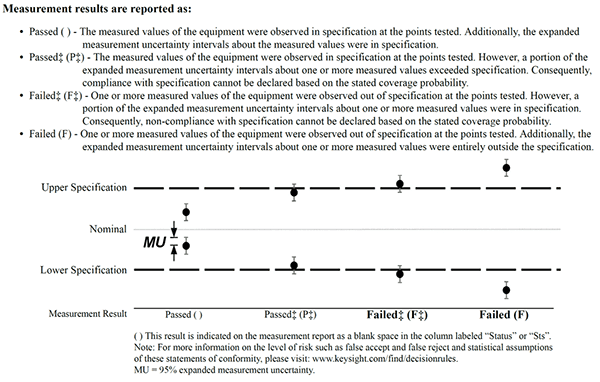
How to Implement These Decision Rules in Your Laboratory
If you want to implement something similar, look at the statement below. You can simply copy and paste this in your test or calibration reports to help you meet the new ISO/IEC 17025 requirements.
- PASS – Results ± expanded uncertainty are within limits/specifications
- PASS‡ – Results ± expanded uncertainty are within but overlap limits/specifications
- FAIL‡ – Results ± expanded uncertainty exceed but overlap limits/specifications
- FAIL – Results ± expanded uncertainty exceed limits/specifications”
Example 2: Not Taking Uncertainty into Account (Simple Acceptance)
In this example, you will see how Vaisala provides statements of conformity without taking measurement uncertainty into account.
In the image below, you will see that their calibration reports state:

In the next image, you will see how Vaisala reports statements of conformity (e.g. “PASS” or “FAIL) in their calibration reports. If you look at the below the results, you will notice Vaisala’s decision rules.
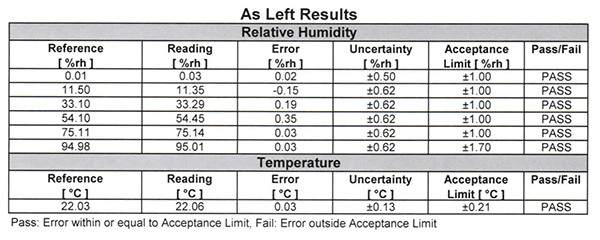
Making statements of conformity without taking measurement uncertainty into account is known as simple acceptance.
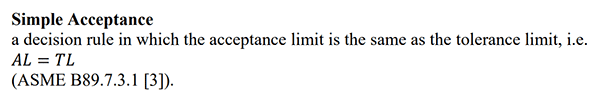
This method is popular. The majority of accredited labs are using simple acceptance. However, some assessors and lab professionals are not fans of the practice. Instead, they believe that ISO/IEC 17025 accredited labs should take uncertainty into account.
Regardless of your opinion or thoughts on this topic, labs are getting accredited using simple acceptance. If you wish to use it in your laboratory, I would prepare yourself for both scenarios:
- taking uncertainty into account, and
- not taking uncertainty into account
This will at least prepare you for several situations, including:
- demonstrating competency and objective evidence during an assessment,
- capability to provide it should customers request it, and
- readiness to implement should policies (on simple acceptance) change in the future.
In another example, see how Epsilon provides statements of conformity without taking measurement uncertainty into account.
In the image below, you will see that their calibration reports state:
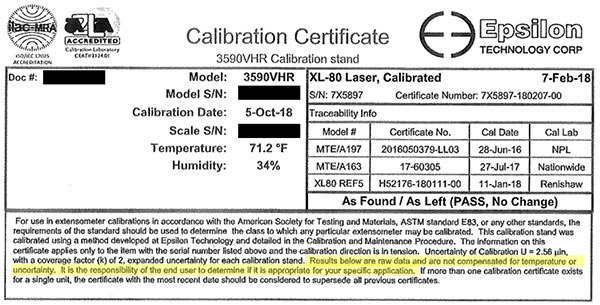
Epsilon states “PASS” in their calibration reports but does not take measurement uncertainty into account when making this statement of conformity.
Again, this practice is not accepted by everyone, but you can use it if you document your decision rules properly and communicate this to your customers as part of contract review. If you include this information in your quotes or service agreements and customers decide to still do business with you (e.g. issue a purchase order, sign a contract, etc.), they are accepting this practice.
Additionally, make sure your customers are not complaining that you are not taking measurement uncertainty into account. If you are receiving a lot of customer complaints, you may receive a deficiency during an assessment.
How to Implement These Decision Rules in Your Laboratory
If you plan to provide statements of conformity without taking measurement uncertainty into account, then you may want to add a statement to your test or calibration reports that is similar to the statement below;
- PASS – Results within limits/specifications
- FAIL – Results exceed limits/specifications”
Example 3: Simple Acceptance Taking Uncertainty into Account
If you are accredited with A2LA or UKAS, you may (or may not) be familiar with their interpretation of the simple acceptance definition. You can learn more about this by reading the following documents:
- A2LA G136: Guidance on Decision Rules in Calibration
- UKAS LAB 48: Decision Rules and Statement of Conformity
To make this short, you need to take uncertainty into account even if you are using simple acceptance.
How you take it into account is up to you. However, both the A2LA G136 and the UKAS LAB 48 have examples for what is acceptable and not acceptable. For reference, the UKAS LAB 48 has a lot more information and examples.
To meet the requirements of A2LA or UKAS, look at the examples below. These are simple solutions you can copy and paste into your test or calibration reports.
The image below gives the examples shown in A2LA G136.
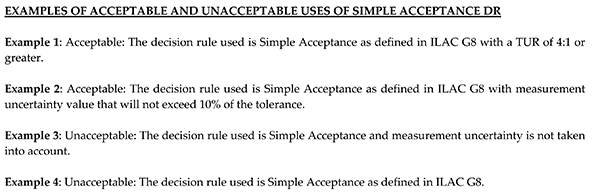
The example below is consistent with A2LA G136 and meets R205, Section 4.4, #3.
The image below gives examples from UKAS Lab 48.
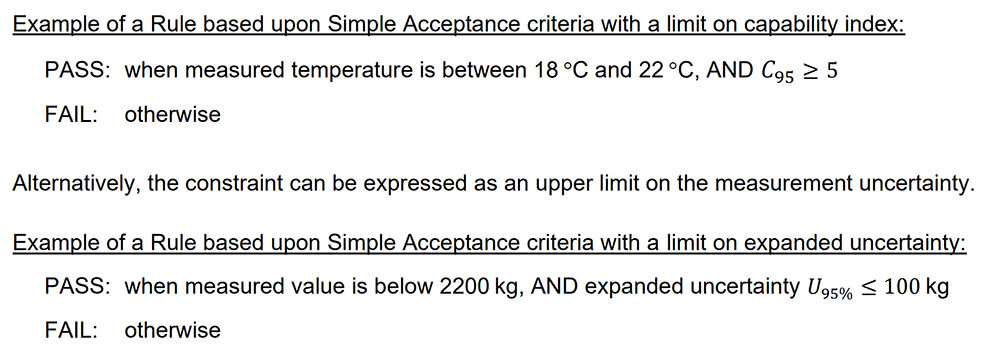
The example below is consistent with UKAS LAB 48.
PASS: when the result is within the tolerance interval and C95 ≥ 4
FAIL: otherwise”
The examples above are simple solutions. There are other options you can use, but these were chosen because they are simple and applicable to many scenarios. This is great for laboratories that have a variety of test or calibration capabilities.
If your laboratory has a smaller scope of accreditation, you may want to use more specific examples given in the UAKS LAB 48.
Example 4: Do Not Report Statements of Conformity
In this example, you will see how Fluke Calibration does not provide statements of conformity. Instead they use symbols to indicate that a result may need to be reviewed further.
In the image below, you will see that Fluke calibration certificates state:

In this image, you will see how Fluke reports calibration results without a statement of conformity.
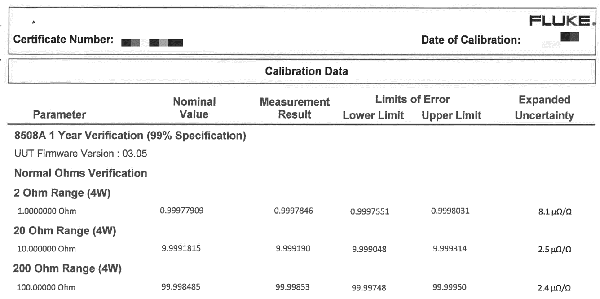
There are other accredited laboratories that provide results without statements of conformity. However, some assessors have interpreted the use of a symbol to identify an out of tolerance result as a statement of conformity. If you want to use a similar process, be careful. You may still receive a deficiency.
Since publishing this guide, I have to mention that I have observed some Fluke calibration reports that do take uncertainty into account and provide statements of conformity similar to the Keysight example in the previous section. I will try to get a copy of one of these reports to give you another example.
How to Implement These Decision Rules in Your Laboratory
If you do not want to provide statements of conformity in your test or calibration reports, consider adding the statement below to your certificates. Remember, do not provide any information in your reports that may be considered a statement of conformity.
Communicating Decision Rules Your Customer
Finally, make sure that you communicate your decision rules to your customers. It is an ISO/IEC 17025 requirement.
ISO/IEC 17025:2017 Requirement

Section 7.1.3 of the ISO/IEC 17025:2017 standard states:
How to Implement These Decision Rules in Your Laboratory
You need to incorporate this into your contract review process; or else, you will not meet ISO/IEC 17025 requirements.
Therefore, it is very important that you include a note or disclaimer in your quotes, proposals, contracts, etc. to clearly communicate your decision rules to your customers.
If they provide you with a purchase order or payment in reference to one of your quotes, they are effectively agreeing to your decision rules. This is the easiest way (in my opinion) to meet this requirement.
If you are a laboratory that is part of a larger company where your company is your customer (i.e. you do not accept outside work), consider creating a service or operating agreement (between the lab and the departments you provide service to) that communicates your decision rules and other requirements. This will give you objective evidence to show you communicated your decision rules to your customer.
However, you should be aware that this is not a fool-proof process of communicating decision rules to your customers. You need to make sure that your customers are not complaining about your decision rules or statements of conformity (or lack of).
If you have customers that complain about your decision rules after services are rendered, you may have a problem that could result in a deficiency during an assessment. I have observed this when reviewing audit results. So, assessors may check your complaint log if they do not like your process.
Still, consider the bright side of your complaint log. If an assessor does not like your process of communicating decision rules using a disclaimer in your quote and you have no complaints, then consider using your complaint log as objective evidence that your customers have agreed to your decision rules.
Conclusion
Statements of conformity and decision rules are two topics of the ISO/IEC 17025 standard that have caused problems for a lot of laboratories who are seeking solutions to meet requirements. Even though there are guides and training available, most of them provide solutions that are too advanced or confusing for what most laboratories (and their customers) need.
In this guide, you should have learned all about statements of conformity and decision rules; and, some simple solutions to help you meet requirements.
Review some of the options and examples given to you in this guide and decide which option will work best for your laboratory. Next, update your quality management system, quotes, and certificates. Finally, implement the process that you have chosen and monitor your results to see if it is effective for your laboratory and customers.
If it is effective, congratulations! If it is not effective, revise your process until it is effective.
What option do you use?
Leave a comment below and let me know.
Originally posted: January 14, 2020
Post Updated: July 14, 2022



