
Introduction
A laboratory’s scope of accreditation is a key element of ISO/IEC 17025 accreditation and a vital asset to your customers. It lists all of the activities that your laboratory is accredited perform.
However, many laboratories forget to consider its importance when getting accredited or reaccredited. They just rush to develop a scope of their capabilities and submit it to their accreditation body.
Instead, you need to make sure to develop a scope of accreditation that is functional for both your laboratory and customers.
It is a great marketing tool that make an impact your business.
When a customer looks at your scope, they want to know one or more of the following;
1. Is your laboratory ISO/IEC 17025 accredited,
2. Can you calibrate/test “x,”
3. What is your measurement uncertainty. and(or)
4. What methods are you using to calibrate/test “x?”
If your scope of accreditation does not answer these questions, you may have a problem that could be driving away potential customers.
In this guide, I am going to show you a simple five step process that will help you develop an amazing scope of accreditation for your laboratory. Plus, I have included plenty of important links to valuable resources that will help you meet ISO/IEC 17025 requirements.
So, if you are looking to create or update your scope of accreditation, let’s get started.
Background
When I took over a calibration program for an accredited laboratory, I remember reviewing and updating the scope of accreditation for the first time.
It wasn’t difficult. Most the work had already been done for me.
However, when I needed to add new capabilities, I always had a tough time figuring out the right terminology and format to use.
So, I read the policies, requirements, and guides published by my accreditation body. Yet, I still struggled to get it right. Most of the guides were great for knowing the rules, but did not provide exact match examples that met my needs.
Therefore, I started to look at other laboratories’ scopes of accreditation for inspiration.
It was great! I was able to review several laboratory scopes that were similar to mine and replicate the format that I thought would fit my laboratory’s needs.
But meeting the needs of my laboratory was not enough. I had to learn the hard way that our scope of accreditation was not helping our current and prospective customers.
At the time, we were receiving a ton of phone calls each week with questions about our calibration capabilities. We were helping customers by answering their questions, but we were wasting our valuable time and not pushing out quotes fast enough (because we were constantly on the phone).
Now, most people would recommend that we hire more personnel to handle the volume of work. However, I disagree.
We did not have a personnel problem, we had a communication problem. We were not answering our customers’ questions with our scope of accreditation. Therefore, they had to call us to get answers to their questions.
Luckily, they were interested enough in our services to pick up the phone and call!
How many potential customers do you think we were losing because our scope did not answer their questions?
I am not certain; but, I bet it was a lot!
I am confident that the majority of potential customers looked at our scope, did not find the answer they were looking for, and moved on to look at other laboratories’ scopes of accreditation.
Bummer! Another customer lost.
Then, it dawned on me. I needed to design and format my scope to meet my customers’ needs. So, we spent time documenting customer questions and identifying the questions that we the most repetitive.
With this information, I decided to change the terminology used in our scope of accreditation to meet our customers’ needs (i.e. answering questions).
As a result, we were able to reduce the amount of phone calls related to questions and respond quicker to customer inquiries for quotes.
The content of our telephone conversations changed from “Can you calibrate X” to “Can I get a quote for calibration of X.” Furthermore, we noticed a significant bump in the number of requests for quotes (both via telephone and email).
With this in mind, what are potential customers seeing when they look at your scope of accreditation; Do they find what they are looking for or are they moving on to another laboratory’s scope of accreditation?
Whether you are creating your first scope of accreditation or considering the need to update your current scope, I hope that you find this guide helpful.
In this guide, you will learn;
1. What is a Scope of Accreditation
2. Testing Labs vs Calibration Labs
3. Guides For Creating A Scope of Accreditation
4. How to Create a Scope of Accreditation (Step-by-Step)
5. Scope of Accreditation Examples
What is a Scope of Accreditation
According to the ILAC G18, a scope of accreditation is the official and detailed statement of activities for which the laboratory is accredited.
Basically, it is an official list of tests and/or calibrations that your laboratory is accredited to perform.
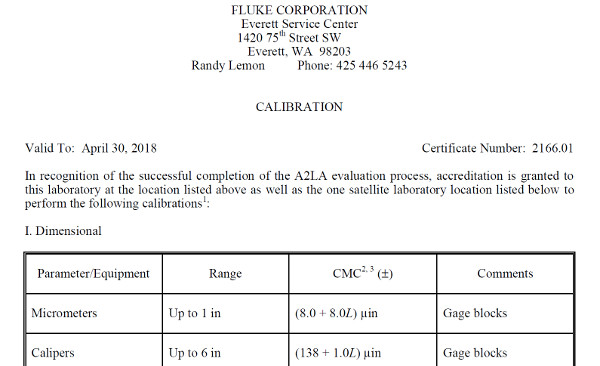
Look at the image below. It is an excerpt of Fluke’s Everett Service Center’s scope of accreditation. If you notice, the scope of accreditation lists the laboratory’s;
• Name,
• Location,
• Technical or Quality Manager,
• Contact information,
• Certificate number,
• Expiration date, and
• List of accredited activities.
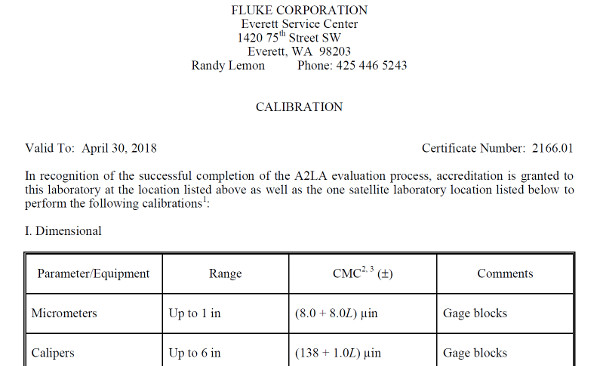
Most laboratories use their scope of accreditation as a marketing tool to showcase their capabilities to current and prospective customers. If you plan to do the same, it is important to ensure that your scope is accurate and up to date.
To provide your customers accredited calibration and test results, the activities must be listed in your scope. If not, you must report the results as non-accredited regardless of capability.
Testing Labs vs Calibration Labs
Every ISO/IEC 17025 accredited laboratory has a scope of accreditation. However, there are slight differences in the presentation of information depending on the type of laboratory.
The scope of accreditation for a testing laboratory is not formatted the same way as a calibration laboratory.
According to the ILAC G18, the scope of accreditation must include:
• Testing Laboratories – The tests or types of tests performed and materials or products tested and, where appropriate, the methods used.
• Calibration Laboratories – the calibrations, including the types of measurements performed, the Calibration and Measurement Capability (CMC) or equivalent.
Look at the image below. You will see a table from the ILAC G18 that lists the typical information included in the scope of accreditation based on laboratory type.
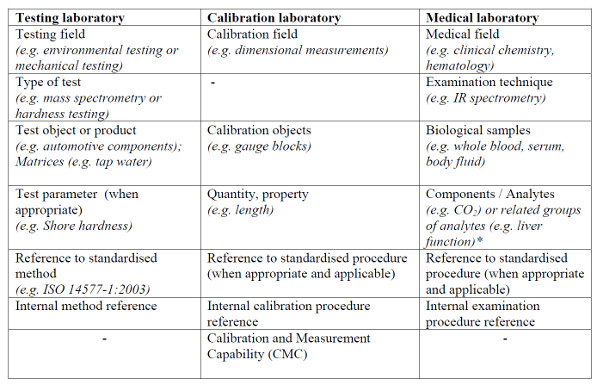
Calibration laboratory scopes typically list the measurement function, range, and CMC Uncertainty for each activity that the lab is accredited to perform. This makes it easy to find and compare laboratory measurement capability and quality.
Testing laboratory scopes typically list only the test activities and methods which they are accredited to perform. Only few testing scopes contain quantitative information about capability and uncertainty.
As a result, the majority of testing laboratory scopes of accreditation do not include statements or estimates of measurement uncertainty. This makes it difficult for customers to compare the capability and quality of testing laboratories.
In an industry of buyer beware, the scope of accreditation should allow customers to confidently find and select laboratories that will meet their requirements; and, provide customers the reassurance that they are conducting business with a laboratory that is ISO/IEC 17025 accredited.
Guides For Creating A Scope of Accreditation
If you are going to create or update your laboratory scope of accreditation, you should make sure that you are meeting ISO/IEC 17025 requirements. Luckily, there are plenty of guides available to help you.
In this section, you will find a list of guides that will help you develop a scope of accreditation.
The first guide that you should read is the ILAC G18. You can access it using the link provided below.
ILAC G18:04/2010 (under revision)

Afterward, you should read your accreditation body’s documents and guides. They will help you meet their specific requirements.
Find your accreditation body from the list below and download the guide relevant to your laboratory type.
A2LA
• G117 – Guidance on Scopes of Accreditation for Testing Laboratories Registered with the US Consumer Product Safety Commission (CPSC)
• G118 – Guidance for Defining the Scope of Accreditation for Calibration Laboratories
ANAB
• PR 2350, Preparing a Draft Scope of Accreditation for ISO/IEC 17025 Testing Laboratories
• PR 2351, Preparing a Draft Scope of Accreditation for ISO/IEC 17025 Calibration Laboratories
PJLA
• Policy on Calibration Scopes of Accreditation PL-4
• Work Instructions for Setting Up Scope of Accreditation Testing WI-8
IAS
• Policy on the Expansion of Scope
NVLAP
• NIST Handbook 150:2016, NVLAP Procedures and General Requirements (PDF)
How to Create A Scope Of Accreditation
Now, that you have some good background information on the scope of accreditation, it is time to get to work and start making one for your laboratory.
All you need to do is follow the five step process listed below to develop your laboratory scope;
1. Contact your Accreditation Body and Get the Draft Template.
2. Read your Accreditation Body’s policies and requirements.
3. Research other laboratory scopes with similar capabilities.
4. Enter your data into the Scope of Accreditation template.
5. Submit it to your accreditation body with your application.
Using this simple five-step process will help you make an amazing laboratory scope for ISO/IEC 17025 accreditation that will benefit both your laboratory and your customers.
If you are ready to get started, just keep reading to learn more about completing each step of the process.
1. Contact Your Accreditation Body and Get the Draft Template.
To create a scope of accreditation, you will need a template. If you have selected an accreditation body and are in the application or reaccreditation process, contact your accreditation body and request a template for your Draft Scope of Accreditation.
The easiest way to do this is send an email to your accreditation officer requesting the template. Typically, they will send it to you within 24 hours. However, if you do not receive the template within 24 hours, follow up with a telephone call.
2. Read Your Accreditation Body’s Policies and Requirements.
Before you begin completing your draft scope of accreditation, read your accreditation body’s policies and requirements. Each accreditation body has their own preferences for how they like scope to be designed. It will help you create your scope using the correct terminology and format.
Additionally, make sure to read your accreditation body’s guides. They will provide you with insight and guidance to prevent you from making mistakes.
Accessing your accreditation body’s documents is easy. You can download them directly from your accreditation body’s website.
To help you out, I have provided a list of accreditation requirements documents below. Find your accreditation body in the list below and click on the link to access the document(s) relevant to your laboratory.
A2LA
• R101 – General Requirements: Accreditation of ISO/IEC 17025 Laboratories
ANAB
• MA 2100, Accreditation Manual for Laboratory-Related Activities
PJLA
• Accreditation Procedure – General – SOP-1 General
IAS
• Accreditation Criteria for Calibration Laboratories (AC204)
• Accreditation Criteria for Testing Laboratories (AC89)
NVLAP
• NIST Handbook 150:2016, NVLAP Procedures and General Requirements (PDF)
3. Research Other Laboratory Scopes With Similar Capabilities.
A great trick to developing your scope of accreditation is to get inspiration from other accredited laboratories. Just look at their scope of accreditation to see how they are listing their activities.
The best part is the information is available online and easy to find. Just search your accreditation body’s search directory. You should be able to find plenty of examples from other laboratories that perform similar activities.
However, do not just look at one scope of accreditation. Try reviewing at least three to five scopes to see how other laboratories list their activities. If you have time, look at ten.
Pay attention to the terminology they use and how they present information. Find examples that you like and replicate it in your scope of accreditation.
While this may seem like a lot of extra work. I promise that it will save you time in the long run. When you are not sure what to do, you can spend a lot of time thinking about how to present the information. Not good!
Always remember that the scope of accreditation should be targeted toward your customers. So, make sure that it answers their questions about your laboratory’s capability.
PRO-TIP: Think of words and copy that will resonate best with your customers. After all, your scope of accreditation is a marketing document. Make sure it designed help customer quickly find what they are looking for!
To search your accreditation body’s directory, use the links provided below.
• A2LA Directory of Accredited Organizations
• NVLAP Directory of Accredited Laboratories
• ANAB Directory of Accredited Organizations
• PJLA Listing of Accredited Labs
• IAS Directory of Accredited Organizations
4. Enter Your Data Into The Scope of Accreditation Template.
Now that you have a template and access to helpful information, it is time take your data and add it to your scope of accreditation.
Be sure to focus on one discipline or measurement function at a time. If you do not, it will be easy to get disorganized and make mistakes. This is especially true if you are developing a large scope with a lot of activities.
With all of the work and stress that you will have during an ISO/IEC 17025 audit, the last thing you want to do is stay at work late fixing mistakes in your scope of accreditation.
The worst mistake that you can make is to forget to add a measurement function entirely. Unless you want to pay for an additional audit, you will have to wait until next time to add new activities to your scope.
So, make sure to pay attention and focus on one measurement function or parameter at a time.
Below, you will find guidance to help you enter data into your scope of accreditation. Read and use the information to develop your laboratory scope.
a. Measurement Function / Parameter / Equipment
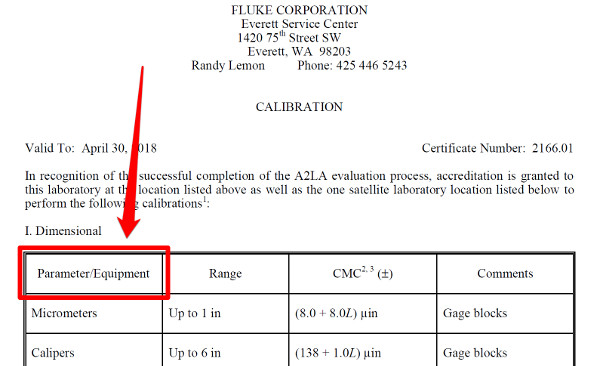
This column defines the measurement functions and disciplines that you are accredited to perform.
You can list functions such as;
• Length,
• Voltage,
• Pressure, and/or
• Temperature.
Or, you can use copy that specifically narrows down your capability to a niche, such as;
• Micrometers.
• Multimeters,
• Pressure Gauges, and/or
• Liquid-in-Glass Thermometers
As you can see, you have some flexibility. However, you will want to check you accreditation body’s requirements to see what type of terminology they will allow.
If you are having trouble deciding, choose the option that will best guide your customer to requesting a quote for calibration services.
b. Measurement Range
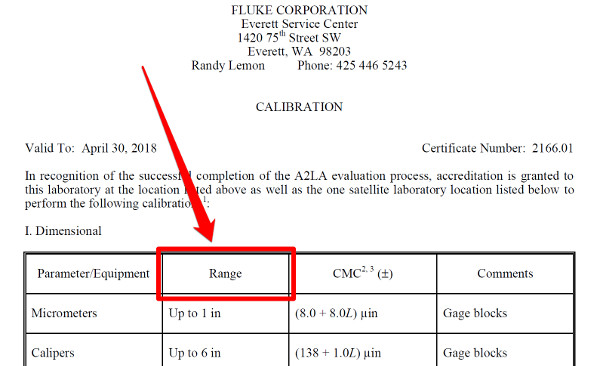
This column defines the range of your measurement function.
Try to think of the minimum and maximum values of you measurement capability. Then, add this information to your scope.
Make sure that you read your accreditation body’s policies, requirements, and guides. Some accreditation bodies will not allow you to use zero (i.e. 0) in your measurement range.
Instead, they may prefer that you use;
• the minimum resolution of the measurement range,
• the phrase “up to” the max value of the range, or
• the minimum value that you would test, measure, etc.
c. CMC Uncertainty
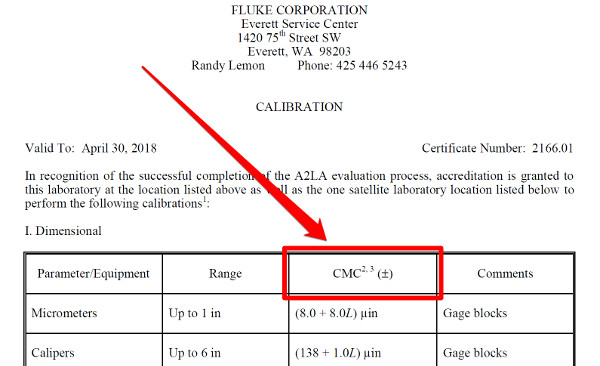
This column is used to define the Calibration and Measurement Capability of your measurement function at a specific measurement range.
Therefore, you will need to list your estimates of uncertainty in measurement for each measurement range included in your scope of accreditation.
When listing your measurement uncertainty, follow the guidance of the ILAC P-14 policy and your accreditation body. Express your CMC Uncertainty as;
• A single value,
• A range of values,
• An equation (typically a linear equation),
• A matrix of values, or
• A graph
d. Comments / Notes / Remarks
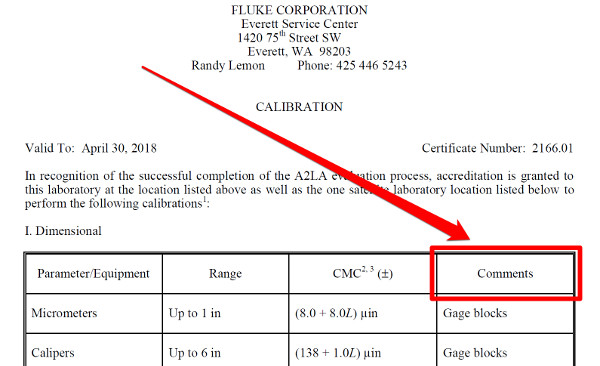
This column is used to provide additional information relevant to your measurement function, range, and CMC Uncertainty.
Some accreditation bodies may use more than one column (e.g. ANAB, IAS, etc) for comments, notes, remarks, etc.
So make sure to follow your accreditation body’s polices and guides. Also, look at other laboratories’ scope of accreditation to find what information they are providing and how it is expressed.
Information typically provided in these sections may include;
• The method,
• The equipment used,
• The location, and/or
• important notes and comments.
5. Submit it to your accreditation body with your application.
Now that you have completed your draft scope of accreditation. Save the file as a Microsoft Word document and email a copy to your accreditation officer.
They will review your scope of accreditation for errors and submit it to your assessors to further review.
If the assessors approve your scope, it will be submitted to the accreditation board for final review.
Once approved, your scope of accreditation will become an official document.
Your accreditation officer will send you a digital and hardcopy version of your scope. Additionally, it will be posted on the accreditation body website so customers can access it via the online search directory.
Scope of Accreditation Examples
In this section, you will see some examples of scopes of accreditation for various accreditation bodies in North America.
Use this information to see how each accreditation body formats their laboratories’ scopes. However, you should still make sure to use your accreditation body’s search directory to look at scopes of accreditation for laboratories that are similar to your laboratory.
A2LA Accredited Calibration Laboratory
A2LA Accredited Testing Laboratory

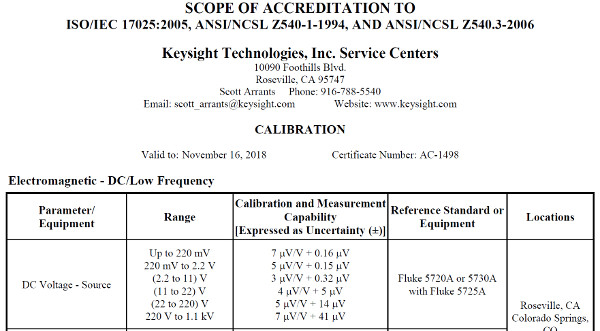
ANAB Accredited Calibration Laboratory
ANAB Accredited Testing Laboratory
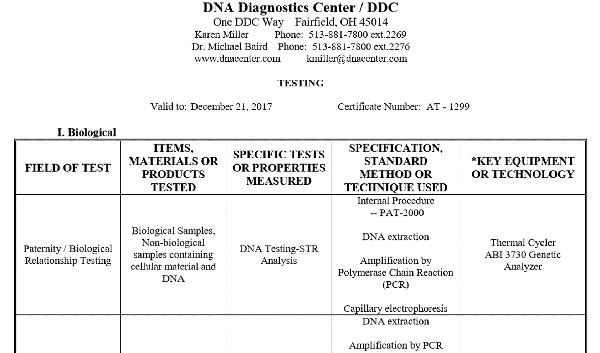
NVLAP Accredited Calibration Laboratory
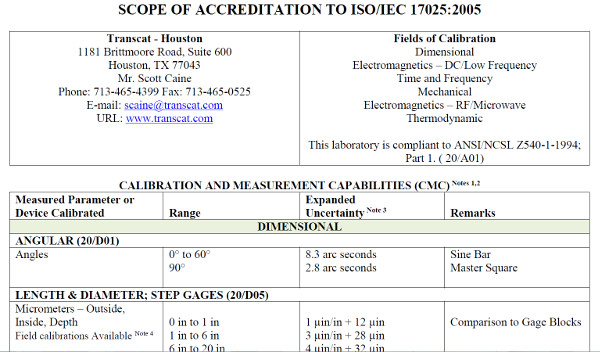
NVLAP Accredited Testing Laboratory

PJLA Accredited Calibration Laboratory

PJLA Accredited Testing Laboratory
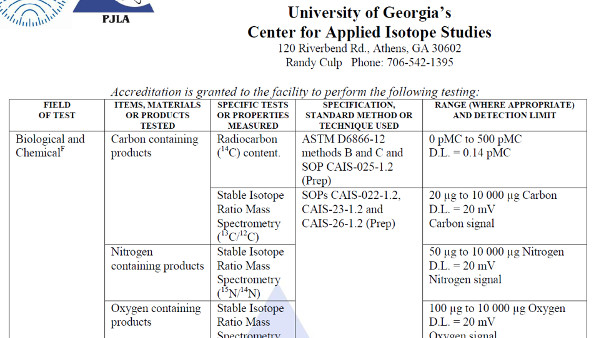
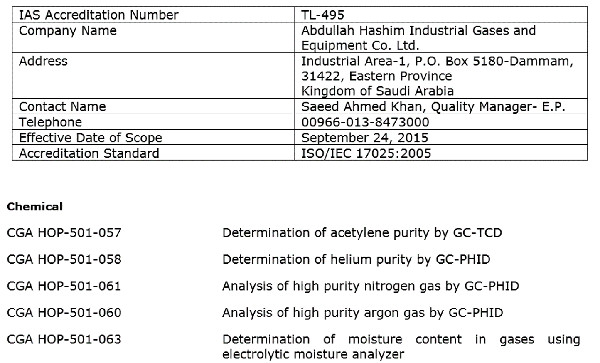
IAS Accredited Calibration Laboratory
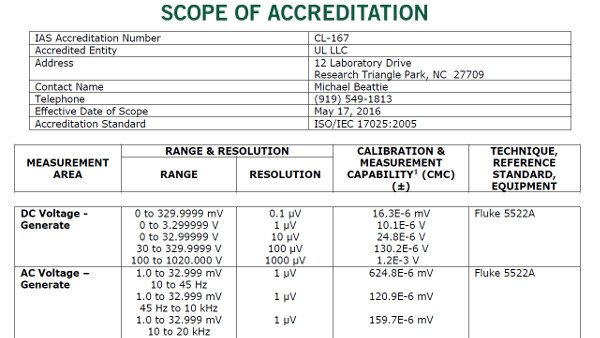
IAS Accredited Testing Laboratory
Conclusion
Developing a scope of accreditation is a key element required for ISO/IEC 17025 accreditation. It lists all of the activities that your laboratory will be accredited to perform.
However, it is also a key element to your business. It is the document that a majority of your customers (requiring accredited testing/calibration services) are going to refer to before doing business with you.
Therefore, it is important that you dedicate some time and effort to developing a scope of accreditation that is functional for both your laboratory and your customers.
Neglecting to do so may negatively affect your business’s reputation and annual revenue.
In this guide, I have given you a simple five-step process to follow when developing your laboratory scope for ISO/IEC 17025 accreditation. Additionally, I have provided you with a ton links to access helpful guides that you can use to ensure that you are meeting requirements.
Whether you are getting accredited for the first time or preparing for reaccreditation, use the information in this guide to develop you scope of accreditation. I promise that it help you create a better scope for your laboratory.
So, give it try and leave a comment below. Let me know your questions, tips, and feedback.




3 Comments