ISO/IEC 17025:2017 Quality Manual Template
$1,499
Complete ISO 17025 Quality Manual Template for all calibration and testing labs. Create a custom quality management system in less than 30 days. Package includes:
- 1 Quality Manual,
- 12 Procedures,
- 13 Lists, and
- 18 Forms
Get the ISO 17025 Quality Manual Template
and be audit-ready in 30 days or less.
Don’t spend 6 months creating a quality manual. Get the ISO 17025 Quality Manual Template and be audit-ready in 30 days or less.
This is a complete quality management system for ISO/IEC 17025:2017, including:
- 1 Quality Manual,
- 12 Procedures,
- 13 Lists, and
- 18 Forms
Just dedicate 1 hour a day to customizing your quality manual template to complete it in 30 days or less.
Why You Should Buy This ISO 17025 Quality Manual Template
Complete Package
This quality management system template includes everything that you need for ISO/IEC 17025:2017, including quality manual, procedures, lists, and forms. All done for you in one bundle.
Easy to Customize
Create a quality manual fast by just filling in the blanks. Each document has fields that you need to complete and includes suggestions and tips to help customize the manual for your laboratory.
Compatible with Most Labs
This quality management system is designed to work withcalibration, mechanical, electrical, dimensional, chemical, automotive, food testing, and Microbiology labs.
Get the ISO/IEC 17025:2017 Quality Manual Template
Create a custom quality managment system and be audit-ready in 30 days or less
BUY THE QUALITY MANAGEMENT SYSTEM TEMPLATE NOWWhy You’ll Love This ISO 17025 Quality Manual Template
It’s Easy to Customize
This quality management system is very easy to customize because it was designed to be that way.
Just Fill in the Blanks
Each document contains brackets and boxes (with suggestions) where you can enter your custom information, such as logos, names, procedures, dates, services, etc. Simply fill in the blanks to make your quality manual for ISO/IEC 17025:2017 accreditation.

Includes Expert Tips and Advice in the Template
If filling the blanks isn’t easy enough, the quality manual includes comment boxes full of helpful suggestions and expert advice to help you customize your quality manual and prepare for ISO/IEC 17025:2017 accreditation.
It’s like having a personal consultant guiding you through the process!
When you are finished customizing your quality manual template, simply delete the comments, and you are done. It is that easy!

It’s Easy to Use: Find Information Fast
Don’t create a quality manual that just collects dust! Get a quality manual that you will actually use.
Seriously! We designed this quality manual template to be user friendly and easy to read. I know that you are wondering how we did it, so let me tell you.
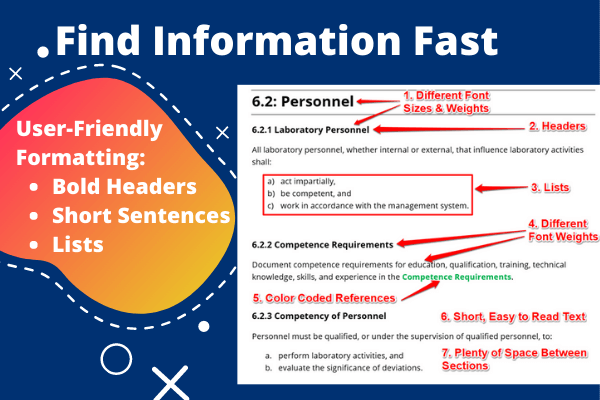
1. Short, Actionable Sentences
First, we removed the long useless paragraphs of passive information that put people to sleep and make finding information difficult.
We replaced them with short, actionable sentences so you know exactly what to do.
2. Bold Headers (Everywhere)
Second, we added bold headers to every section and sub-section of the quality manual so you can scan the manual and find what you are looking for fast!
3. Lists
Third, we replaced long sentences that contain multiple requirements with lists. It seems simple, but it works!
Lists are a great way to find, read, and understand information faster.
Give it try. You will thank me later.
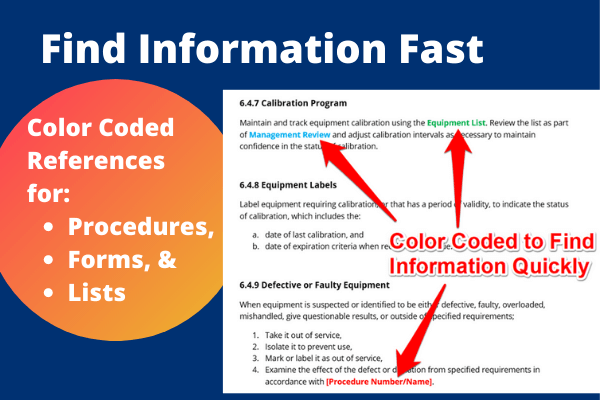
4. Color-Coded Text
Fourth, we added color-coded texts to help you find:
- Procedures,
- References,
- Lists, and
- Forms.
It really makes finding information fast and easy.
It’s Designed for Different Types of Labs
I know that you are thinking, “Will this quality manual template work for my laboratory? We’re not like other labs.”
The answer is, “Yes, it will.”
Made for These Types of Labs
We designed this quality management system to be compatible with various types of laboratories, including:
- Calibration Labs,
- Dimensional Testing Labs,
- Mechanical Testing Labs,
- Electrical Testing Labs (including EMC),
- Chemical Testing Labs,
- Automotive Testing Labs,
- Food Testing Labs,
- and more.
Includes Options for Unique Lab Activities
This quality manual template includes several options to accommodate the different activities accredited labs perform, such as:
- Calibration/Testing,
- Field Testing/Calibration,
- Sampling,
- and more.
Keep the options you need and delete the options that you don’t need. It is very easy.
And, if this quality management system is not compatible with your type of laboratory, we will give a 100% money-back refund.
So, you have nothing to lose.
About this Quality Manual Template
Hi, I’m Rick Hogan.
I made this quality manual template for two reasons:
- To help labs that couldn’t afford to hire me,
- Because I was tired of seeing ugly quality manuals that no one uses (b/c they don’t work).
So, I decided to listen to customer feedback and make a quality manual template that is practical, functional, and easy to use.
The main goal is to have a quality management system that:
- Meets ISO/IEC 17025:2017 requirements,
- Meets laboratory requirements, and
- That lab personnel will actually use.
I know that you may be thinking “That is impossible,” and “No one actually uses the quality manual (except QA).”
Well, I am here to tell you that is not true.
The problem is you don’t have a quality manual that is easy to use and that employees understand. For them, it is easier (and faster) to just ask management (i.e. you).
Trust me! I have been there.
While it feels good to be the person with all the answers, all of these interruptions affect your productivity and profits. Not to mention, how good is your quality management system if you are the only one who knows it. Think about it. What are your personnel doing when you are not around to answer their questions? The answer is whatever they think is the right thing to do.
So, if you do not like getting interrupted 30 times a day to answer questions employees could find in the quality manual (if they just looked), you may want to consider redesigning or reformatting your quality manual.
It could make a BIG difference that…
- Saves you hours of your life,
- Eliminates stress and headaches,
- Improves your productivity, and
- Increases your bottom-line.
After all, you are running a business.
If you think this is impossible, ask me. I have actually managed an ISO/IEC 17025:2017 accredited lab and helped plenty of other labs implement systems that got them accredited. Additionally, I have done this recently; not 20 or 30 years ago before the standard existed.
Real-world experience matters!
Make sure that you consider this when selecting a consultant or buying a solution for your laboratory. Many consultants have ZERO experience operating an ISO/IEC 17025 accredited lab.
What’s Included With theISO 17025 Quality Manual Template
QUALITY MANUAL
Here is a list of the included sections.
- Section 4.1: Impartiality
- Section 4.1: Confidentiality
- Section 5.0: Organization
- Section 6.1: General Resources
- Section 6.2: Personnel
- Section 6.3: Facilties and Environmental Conditions
- Section 6.4: Equipment
- Section 6.5: Metrological Traceability
- Section 6.6: Externally Provided Products and Services
- Section 7.1: Contract Review
- Section 7.2: Selection, Verification, and Validation of Methods
- Section 7.3: Sampling
- Section 7.4: Handling of Items
- Section 7.5: Technical Records
- Section 7.6: Evaluating Measurement Uncertainty
- Section 7.7: Ensuring the Validity of Results
- Section 7.8: Reporting the Results
- Section 7.9: Complaints
- Section 7.10: Nonconforming Work
- Section 7.11: Information Management
- Section 8.1: Management System
- Section 8.2: Management System Documentation
- Section 8.3: Document Control
- Section 8.4: Record Control
- Section 8.5: Risks and Opportunities
- Section 8.6: Improvement
- Section 8.7: Corrective Actions
- Section 8.8: Internal Audits
- Section 8.9: Management Reviews
Procedures
Here is a list of the included procedures.
- SOP 01: Managing Personnel
- SOP 02: Managing Laboratory Equipment
- SOP 03: Intermediate Checks
- SOP 04: Purchasing Products and Services
- SOP 05: Reviewing Contracts
- SOP 06: Validating Methods
- SOP 07: Handling Items
- SOP 08: Ensuring Validity of Results
- SOP 09: Handling Complaints
- SOP 10: Handling Nonconforming Work
- SOP 11: Conducting Internal Audits
- SOP 12: Evaluating Measurement Uncertainty
LISTS
Here is a list of the included lists and logs.
- List 01: Organization Chart
- List 02: Master Document List
- List 03: Distribution List
- List 04: Equipment List
- List 05: Training Log
- List 06: Qualification List
- List 07: Supplier List
- List 08: Purchase Log
- List 09: Method Validation Log
- List 10: PT/ILC Plan
- List 11: Complaint Log
- List 12: LIMS Log
- List 13: LIMS Error Log
- List 14: Risks and Opportunities List
FORMS
Here is a list of the included forms and documents.
- Form 01: Impartiality Agreement
- Form 02: Confidentiality Agreement
- Form 03: Competence Requirements Worksheet
- Form 04: Employee Training Log
- Form 05: Employee Evaluation Form
- Form 06: Purchase Order
- Form 07: Product/Service Inspection Form
- Form 08: Method Validation Form
- Form 09: Complaint Form
- Form 10: Handling Complaints Handout
- Form 11: Nonconformance Form
- Form 12: Customer Survey Form
- Form 13: Corrective Action Form
- Form 14: Internal Audit Checklist
- Form 15: Laboratory Assessment Form
- Form 16: Assessment Finding Form
- Form 17: Management Review Form
- Form 18: Laboratory Temperature & Humidity Log
Get Your Laboratory Ready For Accreditation Buy the ISO/IEC 17025 Quality Manual Template.
This really is a great ISO 17025 quality manual template. It has everything that you need to get ISO/IEC 17025:2017 accredited. All you need to do is download the files and fill in the blanks. Super easy! You’ll be ready for your assessment in just a few weeks.
If you are ready to create a quality management system for your laboratory, click the link below to get your quality manual template today.






Reviews
There are no reviews yet.