ISO/IEC 17025 Internal Audit Checklist
$39
Easily perform internal audits with the ISO/IEC 17025:2017 Internal Audit Checklist. This must-have 22-page PDF checklist:
- is similar to assessor checklists,
- shows you when a procedure or objective evidence is needed,
- highlights the most common deficiencies
Includes 1 Microsoft Word File (.docx) and 1 Adobe PDF File (.pdf).
The Best ISO/IEC 17025 Internal Audit Checklist Available
Do you need to perform internal audits for ISO/IEC 17025:2017 accreditation?
If so, this ISO/IEC 17025 Internal Audit Checklist is for you. This 22-page audit checklist was designed using my favorite elements from the same accreditation body checklists that assessor’s using to audit your laboratory. It does not include the written standard (i.e. ISO/IEC 17025:2017) in it, because you should have a copy of it. However, it does:
- Show you when a procedure, process, or document is needed,
- Indicate when you need to review objective evidence (e.g. records), and
- Highlight the most common deficiencies cited during ISO 17025 assessments.
Truthfully, I like to conduct internal audits using the same assessment checklists used by accreditation bodies. However, this is not always available to everyone. Especially, if you are a laboratory that is applying for accreditation. Therefore, I made this checklist to help you out.
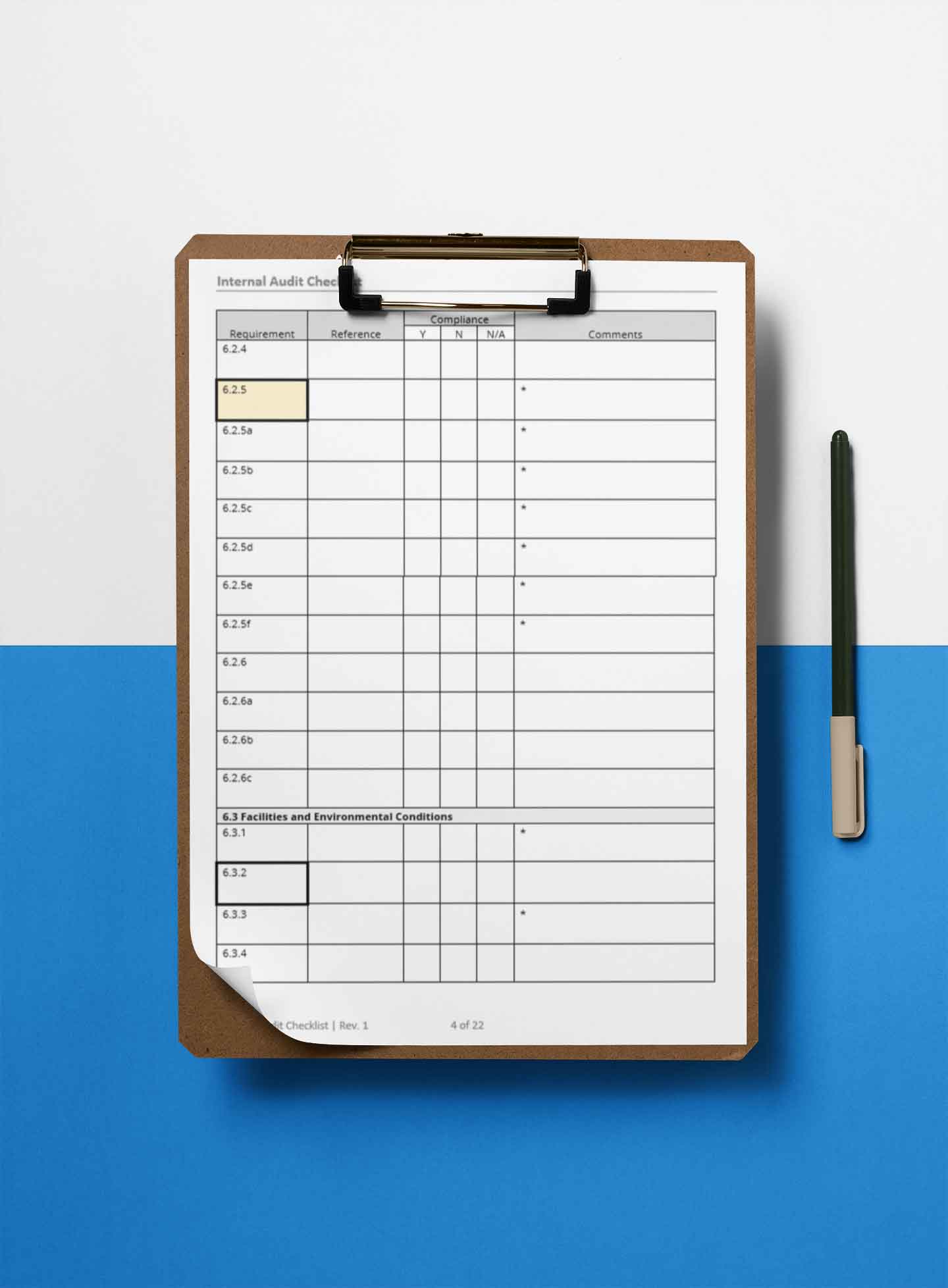
See When You Need A Procedure
When you see a thick, black border, make sure the quality management system has a documented procedure, process, plan, criteria, or other relative document.
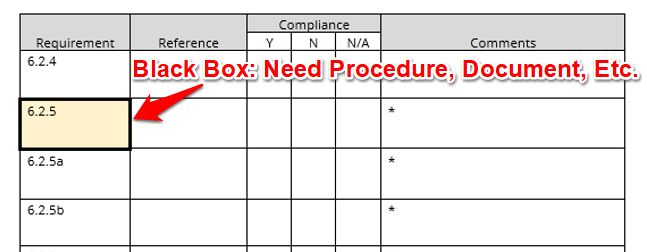
Know When You Need To Review Records
When you see an asterisk (*), make sure that you review records or other objective evidence that support the quality management system and meets ISO/IEC 17025:2017 requirements.

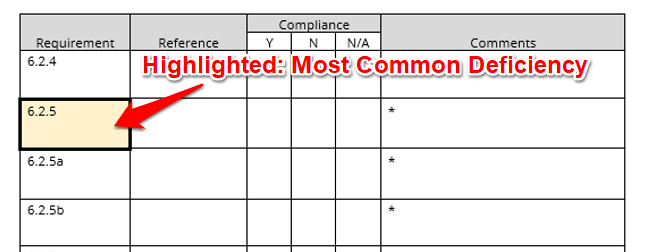
Take A Closer Look At Top Deficiencies
When you see a yellow, highlighted section, you know that this requirement is one of the most common deficiencies cited during ISO/IEC 17025 assessments. Make sure to pay extra attention to these requirements to ensure that your quality management system meets requirements.
Labs Applying for Accreditation
If you are a laboratory that is applying for ISO/IEC 17025:2017 accreditation, I recommend that you perform an internal audit before your assessment.
This will help you:
- Find gaps in your quality management system,
- Reduce the number of deficiencies assessors will find,
- Give you experience performing internal audits, and.
- Help you learn more about your quality management system.
It is best for you to find problems with your management system before an assessor does. It will save you time and money. Fixing deficiencies after an audit requires a lot more documentation and objective evidence (for you to send to the accreditation body) which will cause you to spend more time and money. Trust me, it can be a headache!
That is why I recommend this ISO/IEC 17025 internal audit checklist to you.
Labs That Are Accredited
If your laboratory is already ISO/IEC 17025:2017 accredited, you may not need this checklist. You should already have a checklist, or you can always use the checklist that your accreditation body uses. If you need a copy, just ask them. Or, you may be able to log into your account or portal to download a copy.
However, if you prefer to have your own internal audit checklist, then you may want to consider this checklist. It really is amazing!










Reviews
There are no reviews yet.