THE PERFECT ISO 17025 QUALITY MANUAL TEMPLATE FOR BUSY LABS
-
Don’t Spend 6 Months Creating a Quality Manual. Get Ready for ISO 17025 Accreditation in 30 Days or Less with this Complete Quality Manual Template
Includes:
- 1 Quality Manual
- 12 Procedures
- 14 Lists
- 18 Forms
Buy the Quality Manual Template Now
-

“CREATE A CUSTOM ISO 17025 QUALITY MANUAL IN LESS THAN 30 DAYS”
(Just dedicate 1 hour a day to customizing your manual, procedures, forms, and lists)
-

Easy to Customize
Just fill in the blanks to customize your quality manual. Plus, get expert tips included in the template. -
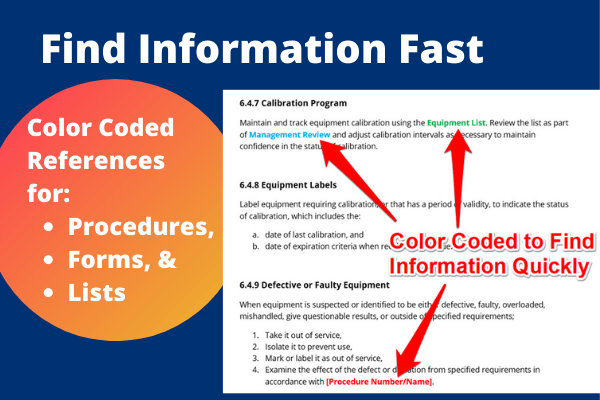
User Friendly
Find information fast with color-coded text, bold headers, different font sizes, and lists. -

Works for Most Labs
Compatible with most types of laboratories: calibration, testing, chemical, & microbiology laboratories.
EVERYTHING YOU NEED FOR ISO/IEC 17025:2017 ACCREDITATION
THE ISO 17025 QUALITY MANUAL TEMPLATE INCLUDES…
-

1 Quality Manual
-

12 Procedures
-

14 Lists and Logs
-

18 Forms
See the Full List
-
Quality Manual
- Section 4.1: Impartiality
- Section 4.1: Confidentiality
- Section 5.0: Organization
- Section 6.1: General Resources
- Section 6.2: Personnel
- Section 6.3: Facilties and Environmental Conditions
- Section 6.4: Equipment
- Section 6.5: Metrological Traceability
- Section 6.6: Externally Provided Products and Services
- Section 7.1: Contract Review
- Section 7.2: Selection, Verification, and Validation of Methods
- Section 7.3: Sampling
- Section 7.4: Handling of Items
- Section 7.5: Technical Records
- Section 7.6: Evaluating Measurement Uncertainty
- Section 7.7: Ensuring the Validity of Results
- Section 7.8: Reporting the Results
- Section 7.9: Complaints
- Section 7.10: Nonconforming Work
- Section 7.11: Information Management
- Section 8.1: Management System
- Section 8.2: Management System Documentation
- Section 8.3: Document Control
- Section 8.4: Record Control
- Section 8.5: Risks and Opportunities
- Section 8.6: Improvement
- Section 8.7: Corrective Actions
- Section 8.8: Internal Audits
- Section 8.9: Management Reviews
-
Procedures
- SOP 01: Managing Personnel
- SOP 02: Managing Laboratory Equipment
- SOP 03: Intermediate Checks
- SOP 04: Purchasing Products and Services
- SOP 05: Reviewing Contracts
- SOP 06: Validating Methods
- SOP 07: Handling Items
- SOP 08: Ensuring Validity of Results
- SOP 09: Handling Complaints
- SOP 10: Handling Nonconforming Work
- SOP 11: Conducting Internal Audits
- SOP 12: Evaluating Measurement Uncertainty
Lists
- List 01: Organization Chart
- List 02: Master Document List
- List 03: Distribution List
- List 04: Equipment List
- List 05: Training Log
- List 06: Qualification List
- List 07: Supplier List
- List 08: Purchase Log
- List 09: Method Validation Log
- List 10: PT/ILC Plan
- List 11: Complaint Log
- List 12: LIMS Log
- List 13: LIMS Error Log
- List 14: Risks and Opportunities List
-
Forms
- Form 01: Impartiality Agreement
- Form 02: Confidentiality Agreement
- Form 03: Competence Requirements Worksheet
- Form 04: Employee Training Log
- Form 05: Employee Evaluation Form
- Form 06: Purchase Order
- Form 07: Product/Service Inspection Form
- Form 08: CMethod Validation Form
- Form 09: Complaint Form
- Form 10: Handling Complaints Handout
- Form 11: Nonconformance Form
- Form 12: Customer Survey Form
- Form 13: Corrective Action Form
- Form 14: Internal Audit Checklist
- Form 15: Laboratory Assessment Form
- Form 16: Assessment Finding Form
- Form 17: Management Review Form
- Form 18: Laboratory Temperature & Humidity Log
-
Meet the Author
Best Practices from 10+ Years of Helping Labs Get Accredited
After listening to customer feedback, Rick created an ISO 17025 quality manual template to help laboratories that couldn’t afford a consultant get accredited.“My goal was to create a complete quality management system for ISO/IEC 17025:2017 accreditation that was easy to use and customize. So easy that my customers could customize their own quality manual and procedures without the need to hire me for consulting.”
-

READY TO CREATE YOUR CUSTOM ISO 17025 QUALITY MANUAL?
(Just dedicate 1 hour a day to customizing your manual, procedures, forms, and lists)